First Treatment for Rare Form of Rickets Approved by FDA
- Updated on: Jun 26, 2024
- 2 min Read
- Published on Apr 23, 2021
New treatment for rare form of rickets
The FDA has approved burosumab, a monoclonal antibody and the first treatment of a rare and inherited form of rickets as announced in a press release by FDA in February 2018. This will be the first global treatment for the rare disease X linked hypophosphatemia.
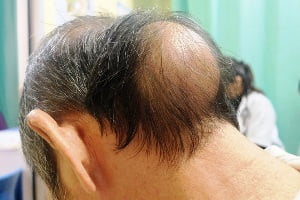
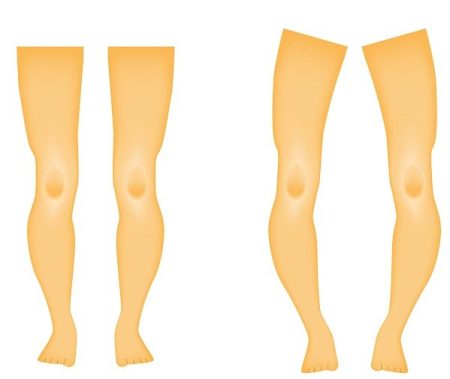
This rare and genetic condition is called X-linked hypophosphatemia, or XLH (hypophosphatemic rickets). It causes low levels of phosphorus in the blood due to excess of fibroblast growth factor 23 production. As a result, bones become weak and impaired and overall bone growth and development suffers in children and adolescents. The disease affects about 3,000 kids and 12,000 adults in the U.S. The disease can affects adults also. Adults with X-linked hypophosphatemia are prone to fractures and bone damage and bone mineralization throughout their lives.
Recommended reading: Problems Due to Calcium Deficiency
What is Burosumab?
Burosumab (KRN23) is an entirely human monoclonal IgG1 antibody that binds excess fibroblast growth factor 23.
Generic Name: burosumab (bur OH sue mab)
Brand Name: Crysvita
In X-linked hypophosphatemia, low phosphate levels in blood are caused by abnormally high levels of FGF23 protein, which causes kidneys to stop phosphate absorption into the bloodstream. Phosphate is essential for the health bones and teeth. Low levels of phosphates in the body can lead to bone deformities and growth problems.
Blocking the FGF23 protein helps the kidneys to maintain normal phosphate levels through appropriate re-absorption into the bloodstream.
Early in 2018 in April, the US FDA approved burosumab for the treatment of XLH in adults and children one year of age and older. This is the first global treatment for the rare genetic form of the rickets. Phase III trials of burosumab are currently being done in adult and child patients with the disease. The drug is also being studied in the phase II trial in adults with tumour-induced osteomalacia and epidermal nevus syndrome globally.
The safety and efficacy of burosumab have been studied in several clinical trials. In a trial, it was found that 94% of adults receiving burosumab once a month achieved normal phosphorus levels as compared to 8% of those receiving placebo. More than 94% of patients treated with burosumab every 2 weeks were able to reach normal phosphorus levels in the body. X-ray reports of adults and children associated with XLH showed improvements with burosumab therapy.
There are however some side effects of the drug when taken for the disease. The most common of these adverse reactions in adults who had taken burosumab included headache, restless leg syndrome, back pain, decreased vitamin D levels, dizziness and constipation. The most common side effects of the drug in children were vomiting, headache, reaction at the injection-site, reduced levels of vitamin D and fever.
FDA has provided intensive guidance to the pharmaceutical company for efficient drug development and review of drugs. Burosumab received orphan drug designation by FDA.